Liliana M. Dávalos, Andrea L. Cirranello, Elizabeth R. Dumont, Stephen J. Rossiter, and Danny Rojas A diversity of cranial phenotypes and feeding ecologies characterizes phyllostomid bats. However, many subfamilies share a similar insect-feeding morphotype, apparently stable over millions of generations. Is the diversity of phyllostomid traits adaptive, and does it contribute to species diversity in the family? We evaluate predictions from Simpson’s ecological theory of adaptive radiation, applied to comparative trait analyses since 2000. The shift to higher rates of taxonomic diversification at the base of Stenodermatinae, new skull architecture capable of great bite force despite small size, and a distinctive olfactory receptor profile make this subfamily a radiation by itself. The stenodermatine skull architecture also enables making leaf tents, a new roost adaptive zone that contributes to the diversity of this subfamily. The evolution of distinct trophic adaptive zones characterized by optima in cranial musculature and mechanical advantage shows that stenodermatines are not the only phyllostomid lineage responding to ecological opportunities. Instead, nectarivores have unique cranial traits and occupy their own trophic adaptive zone, while a subset of insectivores together with sanguivores share a similarly high trophic level. There is also evidence of convergent and adaptive evolution in cranial traits associated with similar trophic levels, as well as adaptations coupled with dietary specialization in digestive and renal function. While linked to higher rates of taxonomic diversification only among stenodermatines, these various adaptations demonstrate the response of phenotypic form to diverse ecological function that makes phyllostomid bats unique.
0 Comments
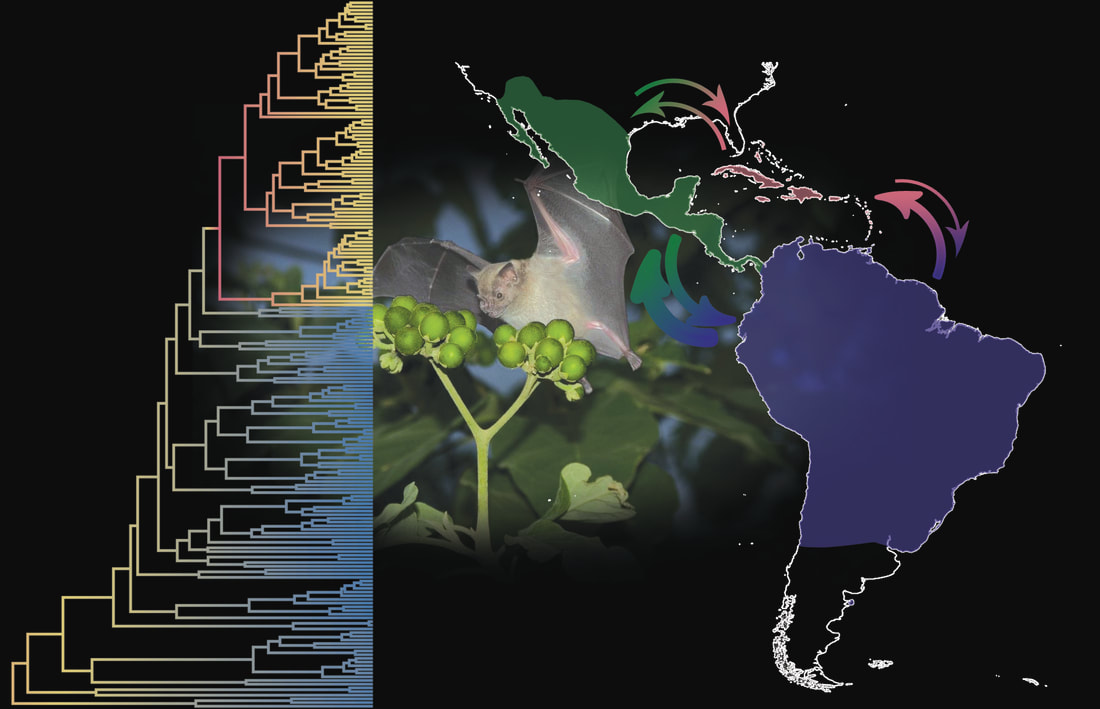
Liliana M. Dávalos, Paúl M. Velazco, and Danny Rojas Traditionally, the phylogeny of phyllostomids contained a few subfamilies sharing similar cranial and ecological traits. Family-wide genetic sequence data starting in the early 2000s, however, showed that this phylogeny obscured the taxonomy, biogeography, and evolution of ecological traits in the family. Current phylogenies strongly support the monophyly of 11 subfamilies, as well as most relationships among them. Nonetheless, the positions of several key groups are not well-supported and require further analyses, including: 1) Micronycterinae relative to Desmodontinae, 2) Lonchorhininae, and 3) the genus-level resolution of Miocene fossils. Historical biogeographic analyses have revealed a South American origin for most subfamilies; have inferred widespread and multi-continental distributions for ancient ancestors; and have rejected a special influence of Quaternary glaciations on recent speciation. These analyses have also confirmed North and Central America, as well as South America, as independent centers of diversification for some subfamilies (e.g., Macrotinae), as well as within subfamilies (e.g., Glossophaginae). These parallel centers of diversity might result from the absence of continuous dry habitat corridors between the continents, but this hypothesis has not been tested using phyloclimatic methods. Biogeographic analyses of individual genera have demonstrated the importance of the Andes for diversification in Platyrrhinus, Sturnira, and Uroderma, as well as the critical role of humid forest corridors between Central and South America for the long-term dispersal of these lineages.
|
Meet the editors!
Theodore H. Fleming, Liliana M. Dávalos, & Marco A. R. Mello Keywords
All
|